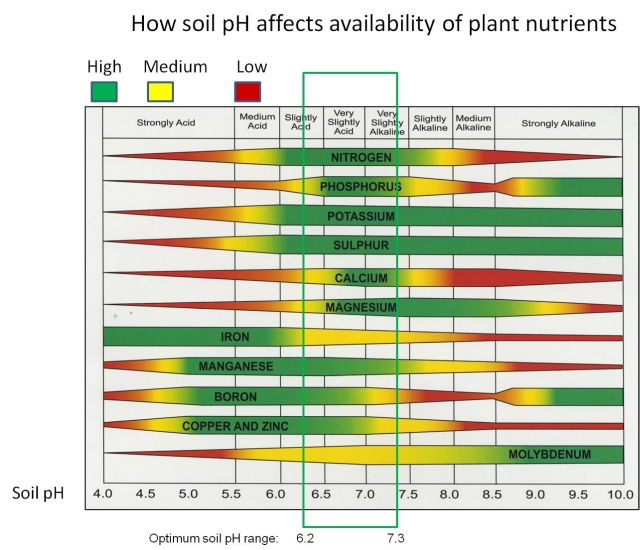
Soil pH plays a crucial role in nutrient uptake by plants. The pH scale ranges from 0 to 14, with values below 7 being acidic, 7 being neutral, and values above 7 being alkaline. Different nutrients are more available to plants at specific pH ranges, and soil pH affects the chemical forms and solubility of these nutrients in the soil. Here’s how soil pH affects nutrient uptake in plants:
- Nutrient Solubility: Soil pH influences the solubility of minerals and nutrients in the soil. Some nutrients are more available to plants in acidic soils, while others are more available in alkaline soils. For example:
- Acidic Soils (pH < 7): Elements like iron (Fe), manganese (Mn), zinc (Zn), and copper (Cu) are generally more soluble and available for plant uptake in acidic soils.
- Alkaline Soils (pH > 7): Nutrients like phosphorus (P), calcium (Ca), and magnesium (Mg) are often more soluble and accessible to plants in alkaline soils.
- Nutrient Availability: Plant roots can only absorb nutrients that are in soluble forms. In soils with a pH that is too high or too low, some essential nutrients can become less available even if they are present in the soil. This can lead to nutrient deficiencies in plants.
- Nutrient Imbalances: Extreme pH levels can lead to imbalances in nutrient availability. For example, in very acidic soils, aluminum toxicity can occur, which hinders root development and nutrient uptake. In highly alkaline soils, micronutrient deficiencies can arise due to reduced solubility.
- Microbial Activity: Soil pH affects microbial activity in the rhizosphere (root zone). Microorganisms play a crucial role in breaking down organic matter and releasing nutrients in forms that plants can absorb. Different microbial communities thrive at different pH levels, influencing nutrient cycling.
- Root Function: Soil pH affects root development and function. At certain pH levels, roots might experience reduced growth or difficulty in penetrating the soil. This can limit the plant’s ability to explore a larger volume of soil for nutrient uptake.
- pH-Dependent Interactions: pH influences various chemical and biological processes in the soil, including interactions between nutrients. For instance, high pH can lead to the fixation of phosphorus in forms that are less available to plants.
It’s important to note that different plant species have varying pH preferences for optimal growth. Some plants thrive in acidic soils, while others prefer alkaline conditions. Understanding the pH requirements of the plants you’re growing and adjusting soil pH accordingly can help ensure proper nutrient uptake and healthy plant growth.
To manage soil pH for optimal nutrient uptake, you can use practices such as liming (raising pH) or adding elemental sulfur (lowering pH) based on soil testing and the specific needs of your plants.